New Pill-Based Treatment for Deadly Sleeping Sickness Now in Use in Five African Countries
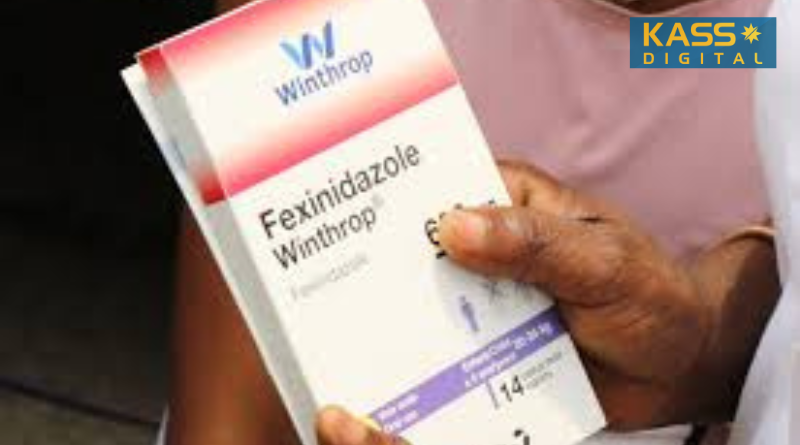
A new pill-based treatment for rhodesiense sleeping sickness, a deadly parasitic disease, is now being used to treat patients in five African countries. The drug, Fexinidazole Winthrop, is the first oral therapy for this fast-progressing form of sleeping sickness, caused by the Trypanosoma brucei rhodesiense parasite.
The new treatment is being rolled out in Ethiopia, Malawi, Tanzania, Zambia, and Zimbabwe. It marks a significant breakthrough, offering an alternative to the previously required intravenous drugs that needed to be administered in hospitals. The treatment can now be taken at home, under minimal supervision, making it more accessible for patients in remote areas.
“This is a safe and simple oral treatment that can be taken at home, revolutionizing care for patients,” said Dr. Westain Nyirenda, principal investigator of clinical trials in Malawi.
Rhodesiense sleeping sickness, transmitted by the tsetse fly, remains endemic to parts of East and Southern Africa, including Kenya. The disease is particularly prevalent in tsetse-infested areas such as Busia and Bungoma counties, parts of Rift Valley, and near national parks and game reserves, where human-wildlife interaction is common.
Although Kenya is not yet part of the rollout, the availability of the drug in neighbouring countries, combined with climate and environmental shifts, makes it highly relevant for Kenyan public health authorities.
“Climate and environmental changes are increasing the risk of outbreaks. We are now better prepared with treatments that can save lives and ease the burden on health systems,” said Dr. Junior Matangila, Head of DNDi’s sleeping sickness program.
The drug was developed through a partnership between the non-profit Drugs for Neglected Diseases initiative (DNDi), the pharmaceutical company Sanofi, and national health programs. It was approved by the European Medicines Agency in December 2023 and added to the World Health Organization’s guidelines in June 2024.
Fexinidazole, which is donated to the WHO by Sanofi’s philanthropic arm, Foundation S, is now being distributed in collaboration with Médecins Sans Frontières. Clinical trials for the treatment were funded by the European Union, the Swiss government, the UK, and other partners.
While Rhodesiense sleeping sickness remains difficult to eliminate due to its animal reservoir, Fexinidazole is the first major advancement in treating the disease in over a decade.
The drug is approved for patients aged six and older, who weigh at least 20 kilograms