First Shipment of Twice-Yearly HIV Prevention Drugs Arrives
By Chemtai Kirui
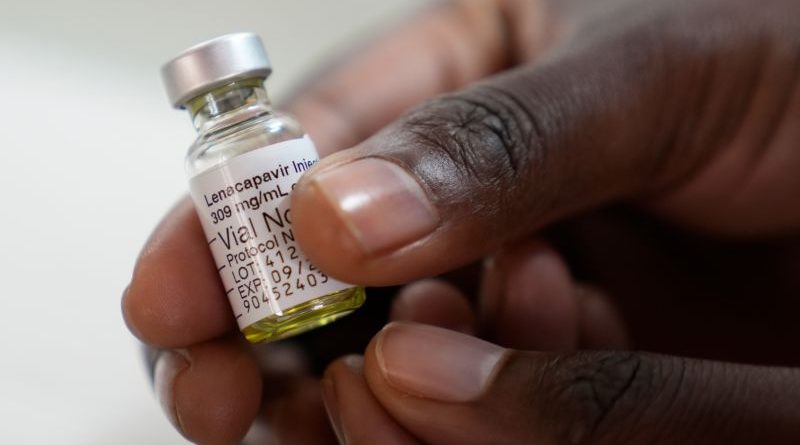
NAIROBI, Feb 18 — The first 21,000 doses of a twice-yearly HIV prevention injection have arrived in the country, marking the start of a phased rollout aimed at reducing new infections in high-burden counties.
The long-acting drug, Lenacapavir, will be introduced beginning March through selected public health facilities in 15 counties where prevalence remains significantly above the national average of 3.7 percent.
Health officials said the injectable, administered once every six months, is expected to address longstanding challenges associated with daily oral pre-exposure prophylaxis (PrEP), including pill fatigue and stigma.
“This shipment represents a significant milestone in our goal to end the HIV epidemic,” Director General for Health Dr Patrick Amoth said during the handover ceremony in Nairobi. “By moving from a daily pill to a twice-yearly injection, we are removing one of the biggest barriers to effective prevention — adherence.”
The annual cost per patient has been reduced to approximately KES 7,800 following negotiated price agreements for low-income countries. Internationally, the drug carries a list price exceeding KES 5 million per year. The service is expected to be provided free of charge in public facilities.
The country currently relies on daily oral Pre-Exposure Prophylaxis (PrEP), typically offered free in public facilities through donor support from the Global Fund and PEPFAR. While effective, uptake has been undermined by pill fatigue and stigma.
Lenacapavir is an antiretroviral medicine, not a vaccine. It works by disrupting the HIV capsid — the protein shell that protects the virus’s genetic material — preventing the virus from entering cells and replicating. The long-acting injection is administered subcutaneously once every six months.
Clinical trial data showed high efficacy in preventing infection among women in southern Africa.
The drug received approval from the U.S. Food and Drug Administration in June 2025 and was endorsed by the World Health Organization the following month. The Pharmacy and Poisons Board registered both oral and injectable formulations for national use in January 2026.
The Ministry of Health, through the National AIDS and STI Control Program (NASCOP), will initially distribute the injection to about 10 facilities per county, including referral hospitals, selected sub-county hospitals and youth-friendly clinics.
The first phase targets Nakuru, Kajiado, Uasin Gishu, Kiambu, Kericho, Nairobi, Kisumu, Homa Bay, Migori, Busia, Kisii, Nyamira, Mombasa, Machakos and Siaya — areas where prevalence remains significantly higher than the national average.

Health workers involved in early introduction efforts say patients may experience injection-site reactions. The drug is administered in the abdomen or thigh and can cause a small, painless lump under the skin that may last several weeks.
Data from the National Syndemic Diseases Control Council showed a 19 percent increase in new infections in 2024, with nearly half of cases recorded among adolescents and young adults. Adolescent girls and young women, who account for about 41 percent of new infections, will be prioritized alongside female sex workers and discordant couples.
Approximately 66,000 doses are expected in the initial shipment cycle. An additional 12,000 doses are due by April to ensure continuity, while 25,000 doses donated by the United States government will support early implementation.
A confirmed negative HIV test on the day of administration is mandatory, as giving the drug to someone already living with HIV risks the development of resistance.
The manufacturer, Gilead Sciences, signed royalty-free voluntary licensing agreements in October 2024 with six generic manufacturers, including firms in India and Egypt. Until generic production scales up, the company is supplying the drug to the Global Fund at no profit to support access in lower-income countries.
Officials said the phased approach will allow authorities to test supply chain systems, including cold-chain requirements, monitor adherence under the six-month schedule, and integrate the injection into maternal health services in areas with high mother-to-child transmission rates.